CDC Biofilm Reactor®
Unlock the Potential of the CDC Biofilm Reactor® for Your Research and Development Needs
Explore the versatile capabilities of the CDC Biofilm Reactor® for a wide range of applications, including product development related to anti-microbial testing, anti-microbial surfaces, and coatings testing, industrial systems corrosion testing, food processing equipment sterilization testing, and medical device testing. Our cutting-edge system is designed to streamline your research processes, making it the ideal choice for scientists, engineers, and academic researchers.
Precise and Efficient Design
The CDC Biofilm Reactor® features eight polypropylene coupon holder rods suspended from a UHMW-polyethylene ported lid. Each holder rod can accommodate three 1/2-inch (12.7 mm) diameter coupons. These components are assembled within a 1-liter glass vessel, complete with a side-arm discharge port. Liquid growth media, biocides, and more can be efficiently circulated through the vessel, with mixing and shear generated by a magnetic stir bar/vane rotated by a magnetic stir plate.
Experience the difference and accelerate your path to innovation and discovery with the CDC Biofilm Reactor®.

CBR 90 Standard CDC Biofilm Reactor®
Includes coupon holder rods, polycarbonate coupons, coupon removal tool, coupon manipulation tool, and glass flow breaks.
Versatility for Comprehensive Research
With the CDC Biofilm Reactor®, you have the power to explore and innovate in diverse areas of research and product development:
- Anti-Microbial Testing: Assess the efficacy of anti-microbial agents and compounds with precision and reproducibility, helping you develop breakthrough solutions to combat microbial threats.
- Anti-Microbial Surfaces and Coatings Testing: Evaluate the performance of anti-microbial coatings and surfaces to create products that promote hygiene and safety in various industries.
- Industrial Systems Corrosion Testing: Study biofilm formation and its impact on industrial systems, enabling you to develop corrosion-resistant materials and practices.
- Food Processing Equipment Sterilization Testing: Ensure the safety and cleanliness of food processing equipment with thorough biofilm testing, essential for maintaining product quality and consumer trust.
- Medical Device Testing: Safeguard the integrity of medical devices by conducting rigorous biofilm assessments, a critical step in ensuring patient well-being and product reliability.
Imaging and Statistical Analysis
Biofilm grown on the coupons can be conveniently imaged directly under a microscope or sampled and plated for statistical analysis, providing you with comprehensive data for your research.
Efficiency and Reusability
The CDC Biofilm Reactor® is designed for ease of use, offering aseptic coupon removal and replacement. This system is autoclavable and reusable, allowing you to maintain consistent, high-quality testing throughout your research journey.
Customization for Your Unique Needs
Choose from a wide variety of coupon materials, including plastics, metals, and ceramics, to tailor your experiments to specific industry requirements. We also offer alternative rod configurations to facilitate the use of porous media and membranes, providing you with even more flexibility to meet your unique research needs.

CBR 90-3 Anaerobic CDC Biofilm Reactor®
Includes seals around each rod and between lid and vessel. The anaerobic CBR includes coupon holder rods, polycarbonate coupons, coupon removal tool, coupon manipulation tool, and glass flow breaks.
Robust Biofilm Growth with the CDC Biofilm Reactor®
Biofilms grown using the CDC Biofilm Reactor® are robust and well-adhered to substrate surfaces. The microscopy images below showcase Pseudomonas aeruginosa biofilms generated using ASTM E3161: Standard Practice for Preparing a Pseudomonas aeruginosa or Staphylococcus aureus Biofilm using the CDC Biofilm Reactor®. The substrate used is a borosilicate glass disc (RD128-GL Borosilicate Glass Disc Coupon), stained with a fluorescent-based live/dead stain to highlight living cells within the biofilm. These images, provided by the Standardized Biofilm Methods Laboratory at the Center for Biofilm Engineering, demonstrate the high-quality biofilm growth achievable with the CDC Biofilm Reactor®.
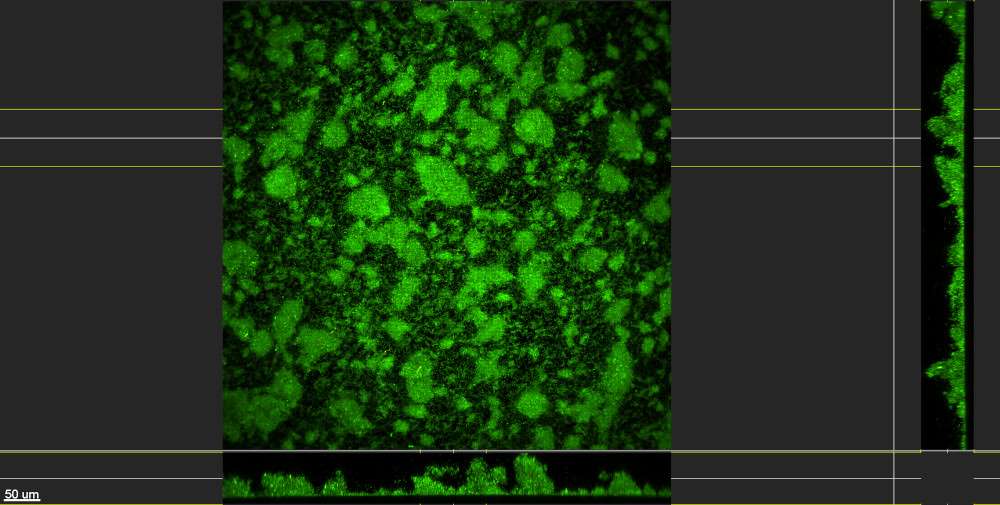
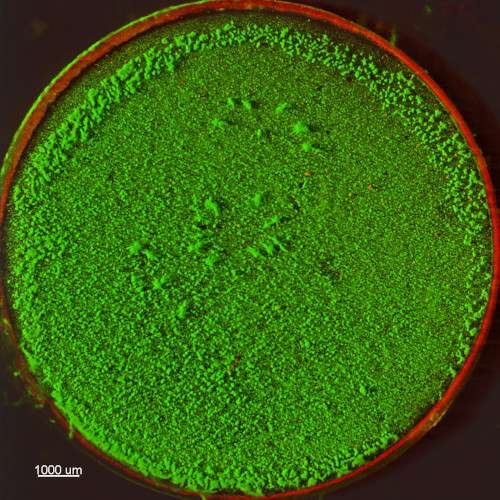
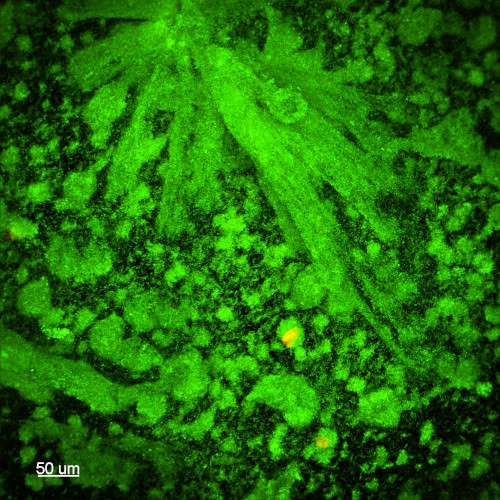
Watch instructional videos on the CBR (Produced by the Center for Biofilm Engineering at MSU):

CBR 2203 Coupon Holder Rod
Polypropylene coupon holder (holds 3 coupons each), 8 included with CDC Biofilm Reactor.

CBR 2203-B Blank Rod
Polypropylene coupon holder rod without coupon recesses used as a place holder after sampling a sample rod (included with CDC Biofilm Reactor).

CBR 2203-GL Slide Holder Rod
Polypropylene rod to hold 0.70 inch (18 mm) wide (or less) x 0.063 inch(1.6 mm) thick slide coupons.

CBR 2203-MBM Membrane Holder Rod
Polypropylene Slide Holder Rod plus polycarbonate porous media/membrane holder cartridge used to capture porous media between 2 membranes/screens, or for transverse membrane studies.

CBR 2203-PM Porous Media Holder Rod
Polypropylene rod with polycarbonate frame to hold membrane samples for tangential flow membrane studies.

CBR 2232 Splash Guard
Insert for preventing splashing inside a 50mL conical vial during sampling.
Required in E2871: Single Tube Method

CBR 2240 Splash Guard
Insert for preventing splashing inside a 250mL conical vial during sampling.
Replacement Parts
All CBR components and parts are available for purchase individually. Check out our full list of replacement parts:
0.5 in. Diameter Sample Coupon
More than 40 materials are already available, and we are always willing to try new materials for specialty projects! Check out our full list of available materials and pricing:
Use in ASTM and EPA Standard Methods
The CDC Biofilm Reactor® is instrumental in adhering to industry standards and best practices, recognized in ASTM and USEPA Standard Test Methods and Guidance:
Standard Test Method for Quantification of Pseudomonas aeruginosa Biofilm Grown with High Shear and Continuous Flow using CDC Biofilm Reactor:
Standard Practice for Preparing a Pseudomonas aeruginosa or Staphylococcus aureus Biofilm using the CDC Biofilm Reactor:
Standard Test Method for Evaluating Disinfectant Efficacy Against Pseudomonas aeruginosa Biofilm Grown in CDC Biofilm Reactor Using Single Tube Method:
Antimicrobial Testing Methods & Procedures: Preparing a Pseudomonas aeruginosa or Staphylococcus aureus Biofilm using the CDC Biofilm Reactor:
Antimicrobial Testing Methods & Procedures: Single Tube Method for Determining the Efficacy of Disinfectants against Bacterial Biofilm:

